Borstkanker
Werkingsmechanisme
Olaparib is een sterke remmer van menselijke poly (ADP-ribose) polymerase-enzymen (PARP-1, PARP-2 en PARP-3) en het is aangetoond dat dit middel de groei van bepaalde tumorcellijnen in vitro en de groei van tumoren in vivo remt, ofwel als opzichzelfstaande behandeling of in combinatie met gevestigde chemotherapieën.1
Samenvattend: BRCAm tumoren hebben een unieke moleculaire biologie die hen gevoelig maakt voor PARP-remming.3,4
Lynparza® remt PARP op twee manieren:*3-5
- Door de enzymatische activiteit te remmen3-5
- Door de vorming van gevangen PARP-DNA complexen te verhogen3-5
In vitro activity does not always correlate with clinical efficacy.
*The exact mechanism of action of LYNPARZA® remains a subject of research.
PARP=poly (ADP-ribose) polymerase; PARPi=poly (ADP-ribose) polymerase inhibitor.
Samenvatting van de resultaten
Vroeg stadium borstkanker (OlympiA studie)
Study design OlympiA
De OlympiA studie onderzocht de werkzaamheid en veiligheid van olaparib als adjuvante behandeling bij patiënten met kiembaan BRCA1/2-mutaties en HER2-negatieve hoog-risico borstkanker in een vroeg stadium, die een definitieve lokale behandeling en neoadjuvante of adjuvante chemotherapie hadden ondergaan.1
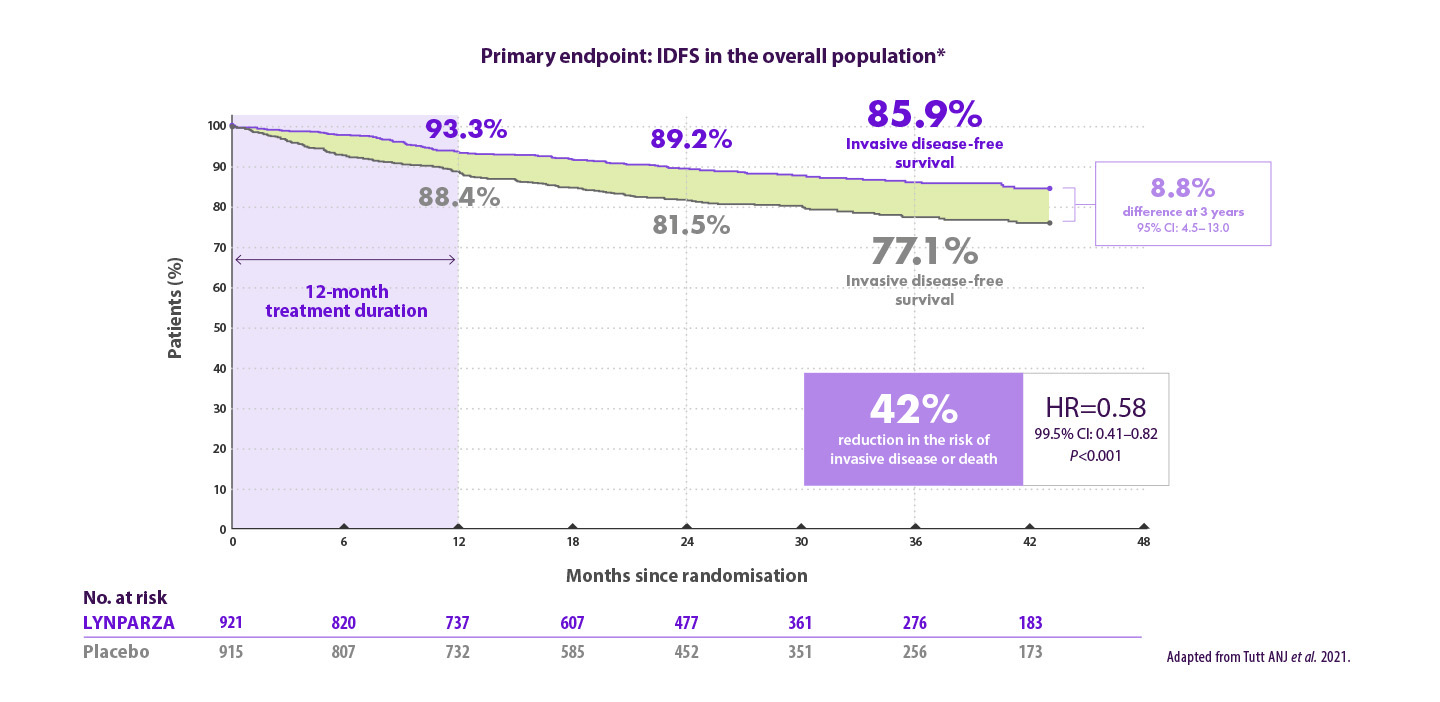
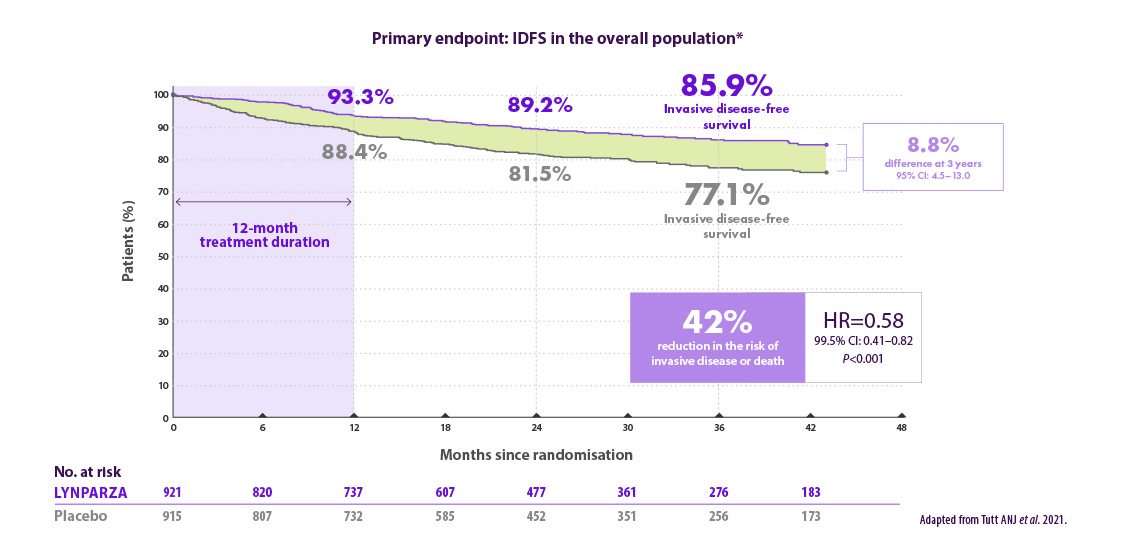
Werkzaamheidsresultaten voor de adjuvante behandeling van patiënten met vroeg stadium borstkanker en een kiembaan BRCA-mutatie in de OlympiA studie
Lynparza® verlaagt het risico op invasieve ziekte met 42% vergeleken met placebo6
*Based on a pre-specified event driven interim analysis with a median follow-up of 2.5 years. CI=confidence interval; HR=hazard ratio; IDFS=invasive disease-free survival.
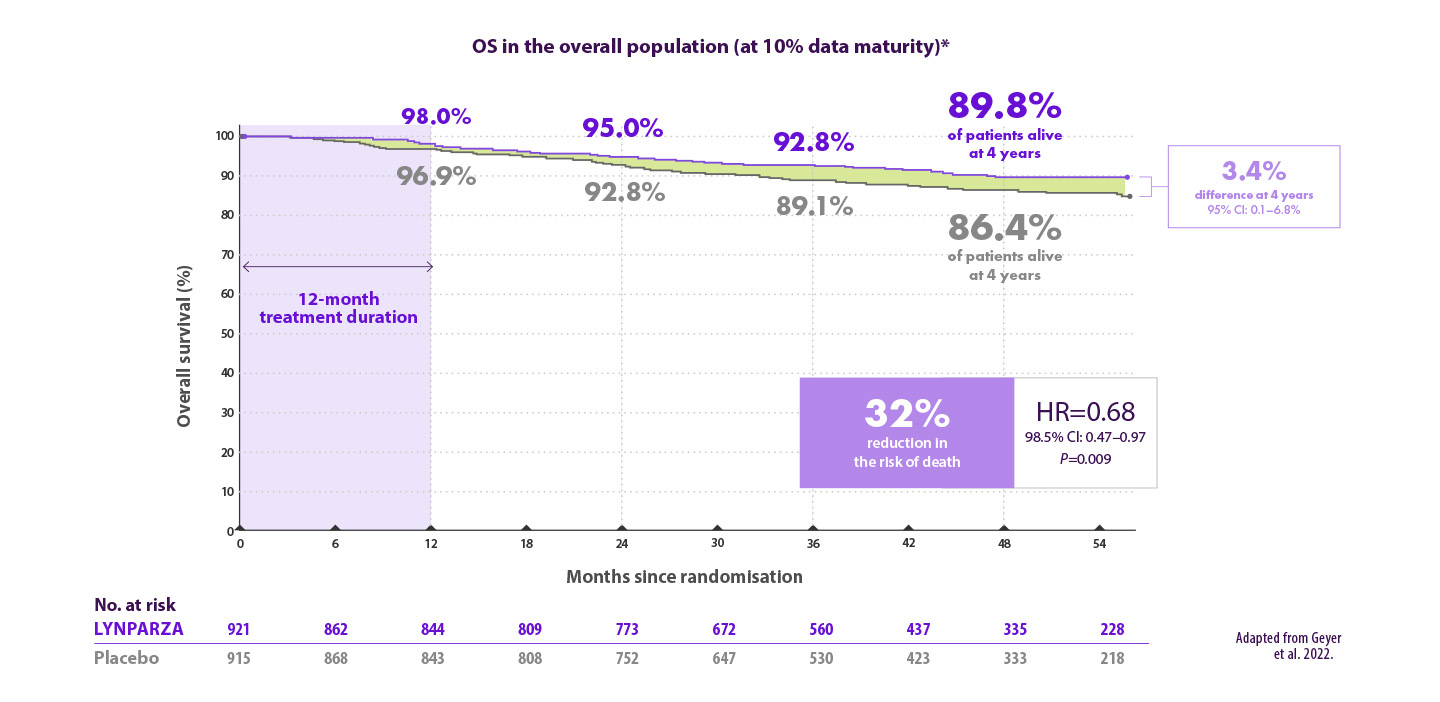
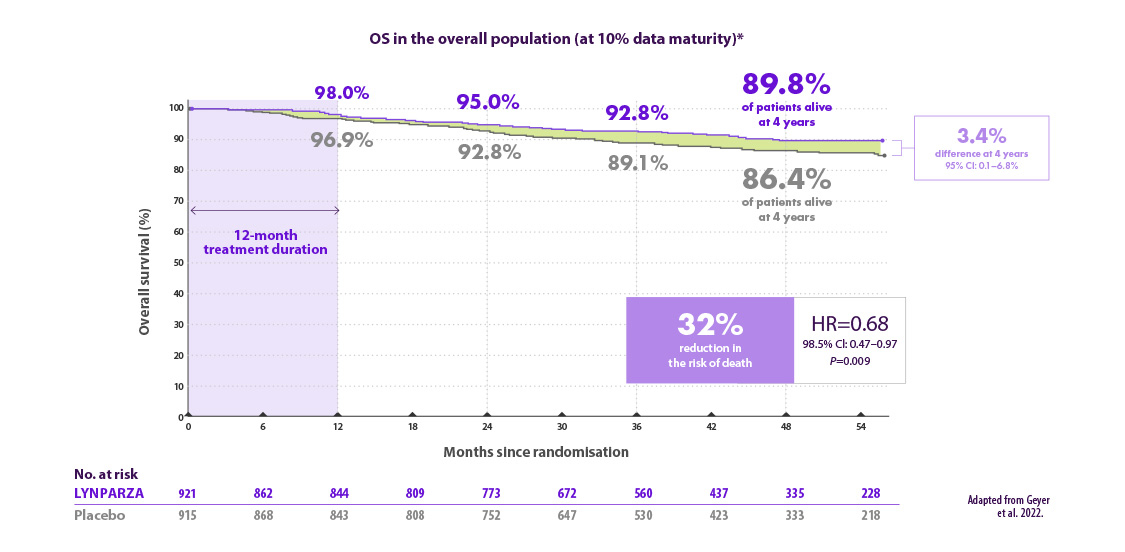
Lynparza® verlaagt het risico op overlijden met 32% vergeleken met placebo7
*OS results are from data cut-off 2, a pre-specified interim analysis with a median follow-up of 3.5 years in the ITT population. CI=confidence interval; gBRCAm=germline BRCA-mutated; HR=hazard ratio; OS=overall survival; ITT=intention to treat..
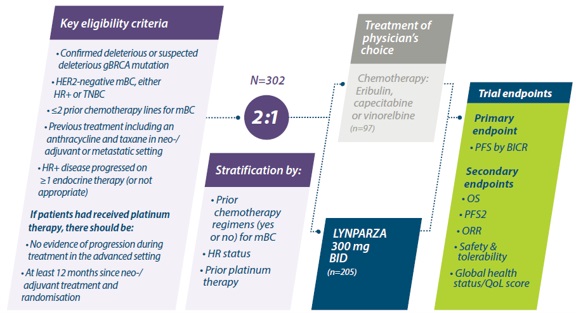
Lokaal gevorderde of uitgezaaide borstkanker (OlympiAD studie)
Study design OlympiAD
De OlympiAD-studie was een gerandomiseerde, open-label fase III-studie waarin olaparib monotherapie werd vergeleken met standaard chemotherapie bij patiënten met een gBRCA-mutatie en menselijke epidermale groeifactorreceptor type 2 (HER2)-negatieve gemetastaseerde borstkanker die niet meer dan twee eerdere chemotherapieën voor gemetastaseerde ziekte hadden gekregen.8
Adapted from Robson M et al. 2017.
BICR=blinded independent central review; gBRCA=germline BRCA; HR=hormone receptor; HR+=hormone receptor-positive; mBC=metastatic breast cancer; ORR=objective response rate;OS=overall survival;PFS=progression-free survival; PFS2=progression-free survival 2 (time to second progession); QoL=quality of life; TNBC=triple-negative breast cancer
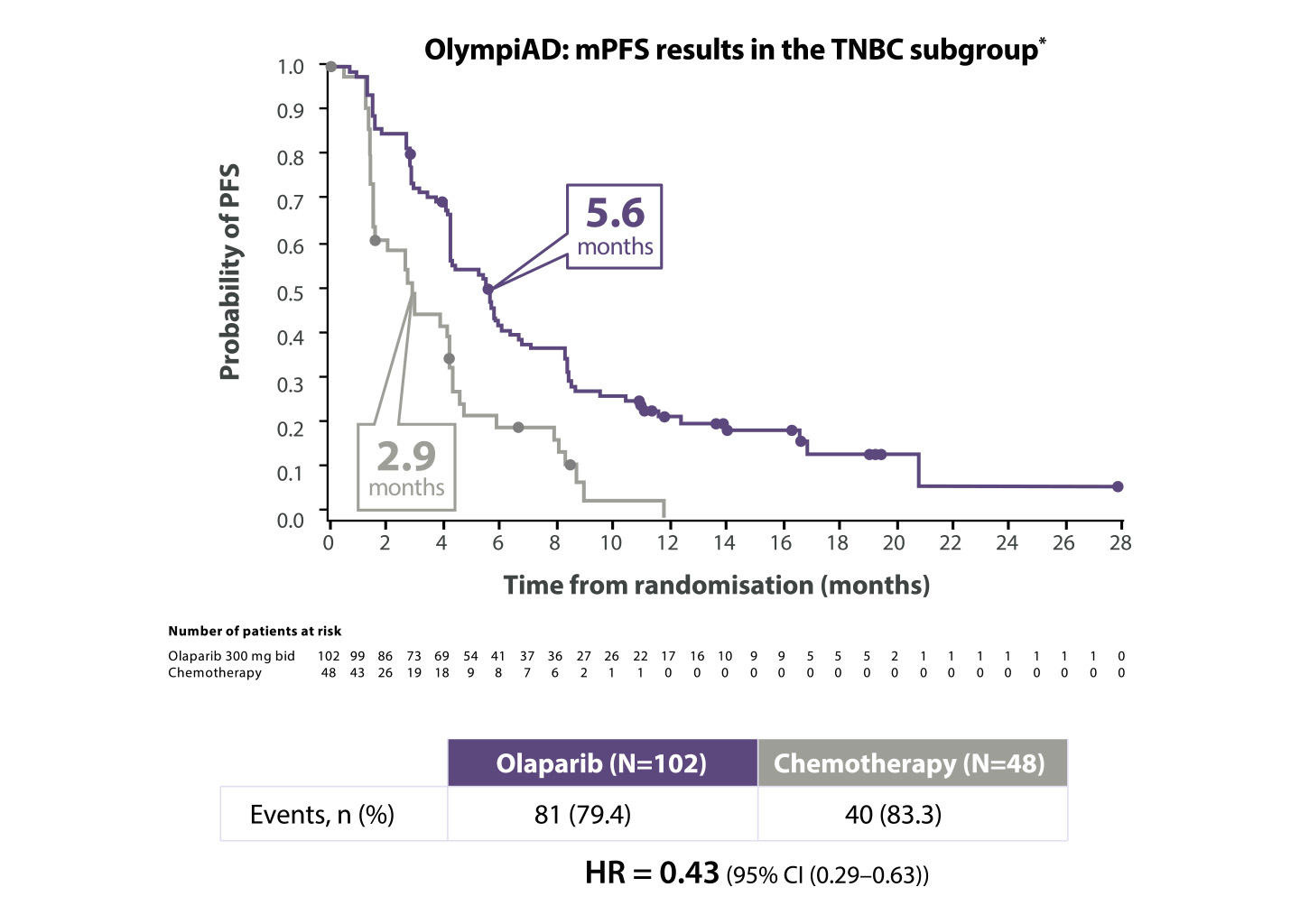
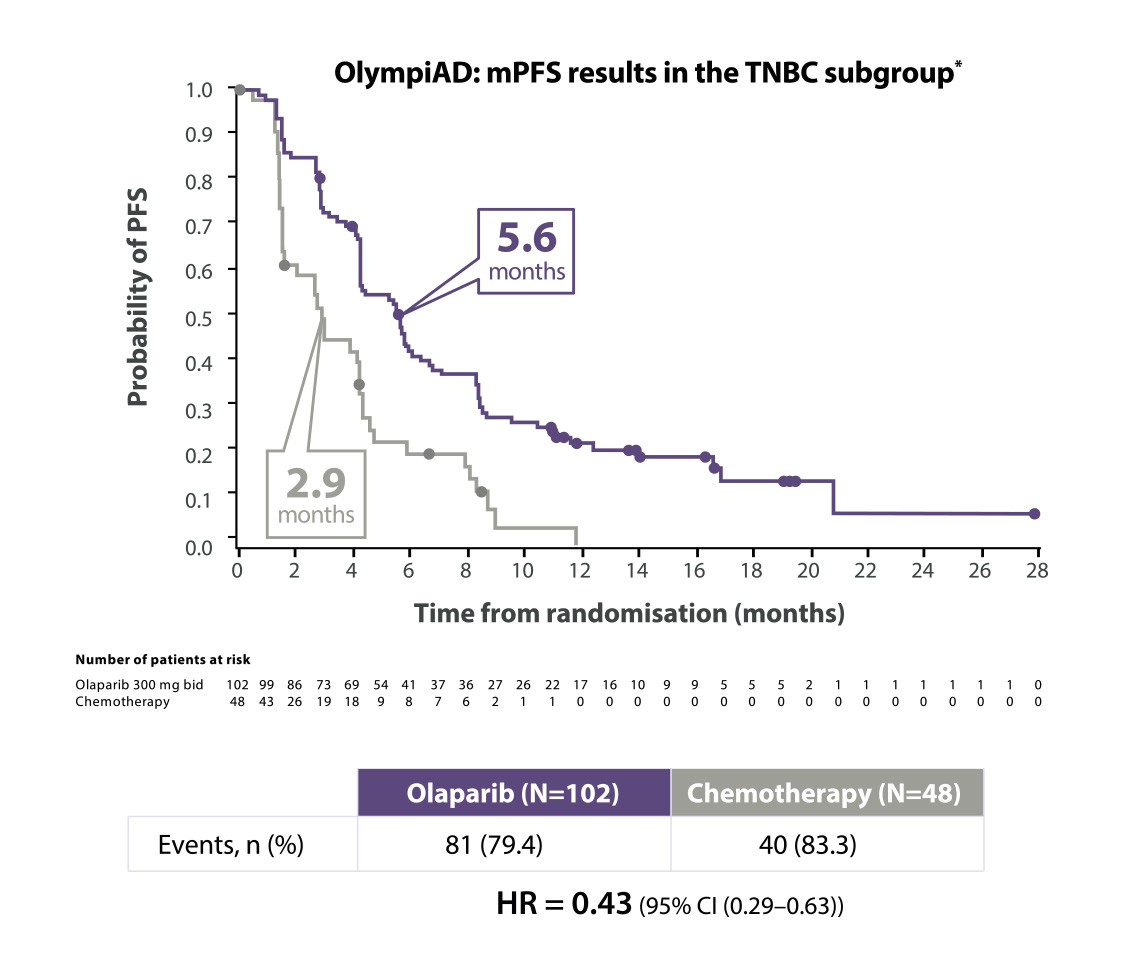
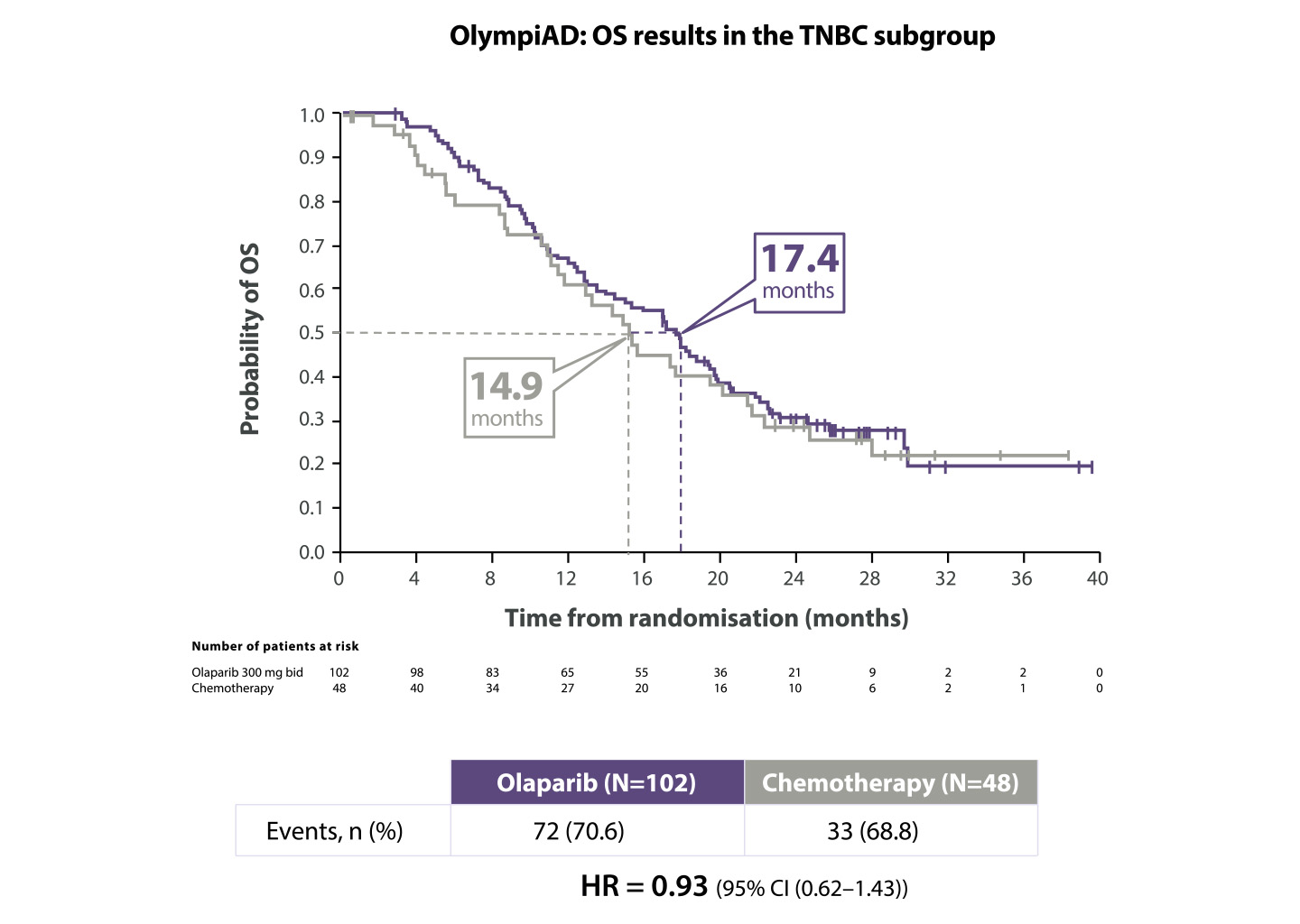
Werkzaamheidsresultaten bij patiënten met HER2-negatieve uitgezaaide borstkanker en een gBRCA1/2-mutatie in de OlympiAD-studie
Lynparza® verdubbelde de PFS in de TNBC-subgroep9
Adapted from Senkus-Konefka et al. 2018
*The OlympiAD study was not powered to identify differences in treatment effect between subgroups, and any differences observed here are hypothesis-generating. Data cut-off: 9 December 2016
BID=twice daily; CI=confidence interval; HR=hazard ratio; (m)PFS=(median) progression-free survival; TNBC=triple negative breast cancer.
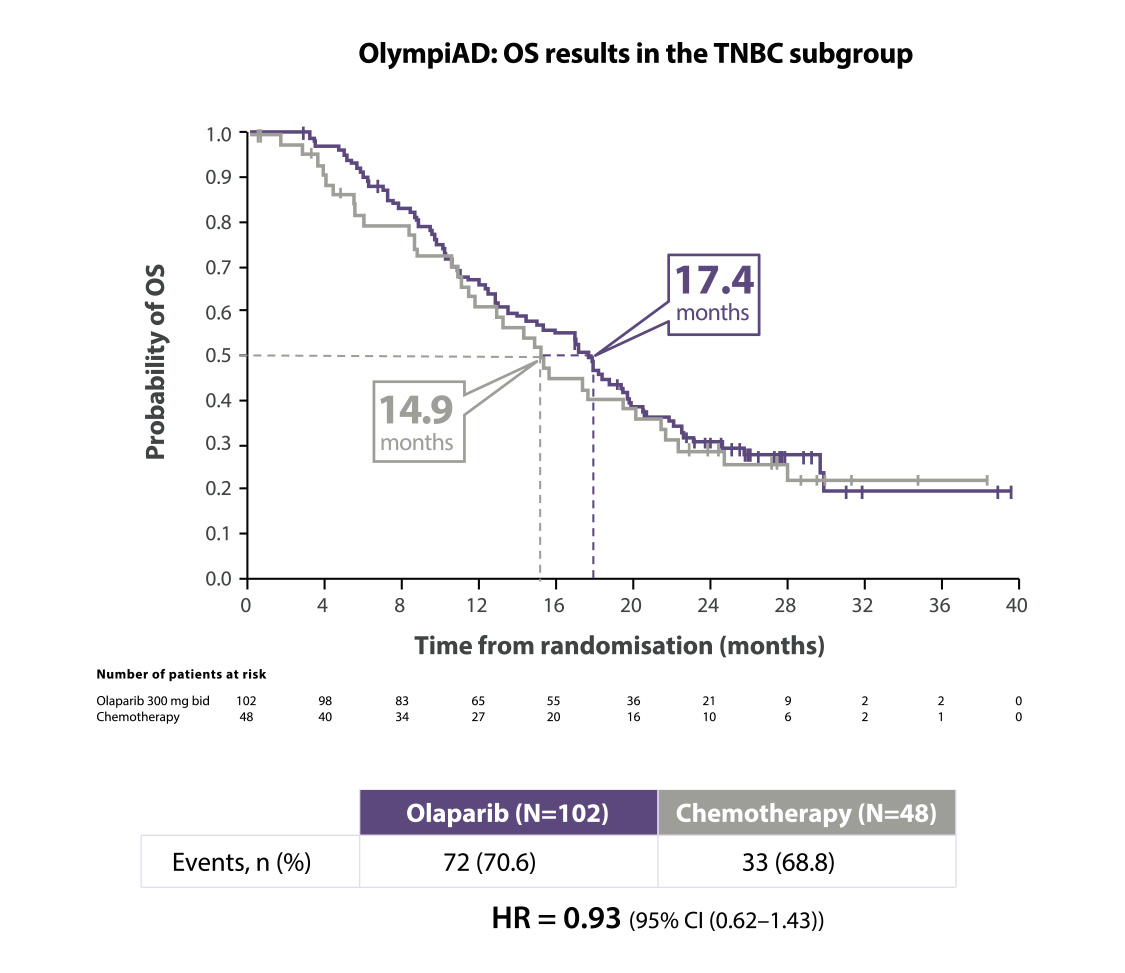
Lynparza®verbeterde de OS in de TNBC-subgroep10
Adapted from Robson M et al. 2019.
*The OlympiAD study was not powered to identify differences in treatment effect between subgroups, and any differences observed here are hypothesis-generating. Data cut-off: 9 December 2016
BID=twice daily; CI=confidence interval; HR=hazard ratio; OS=overall survival; TNBC=triple negative breast cancer.
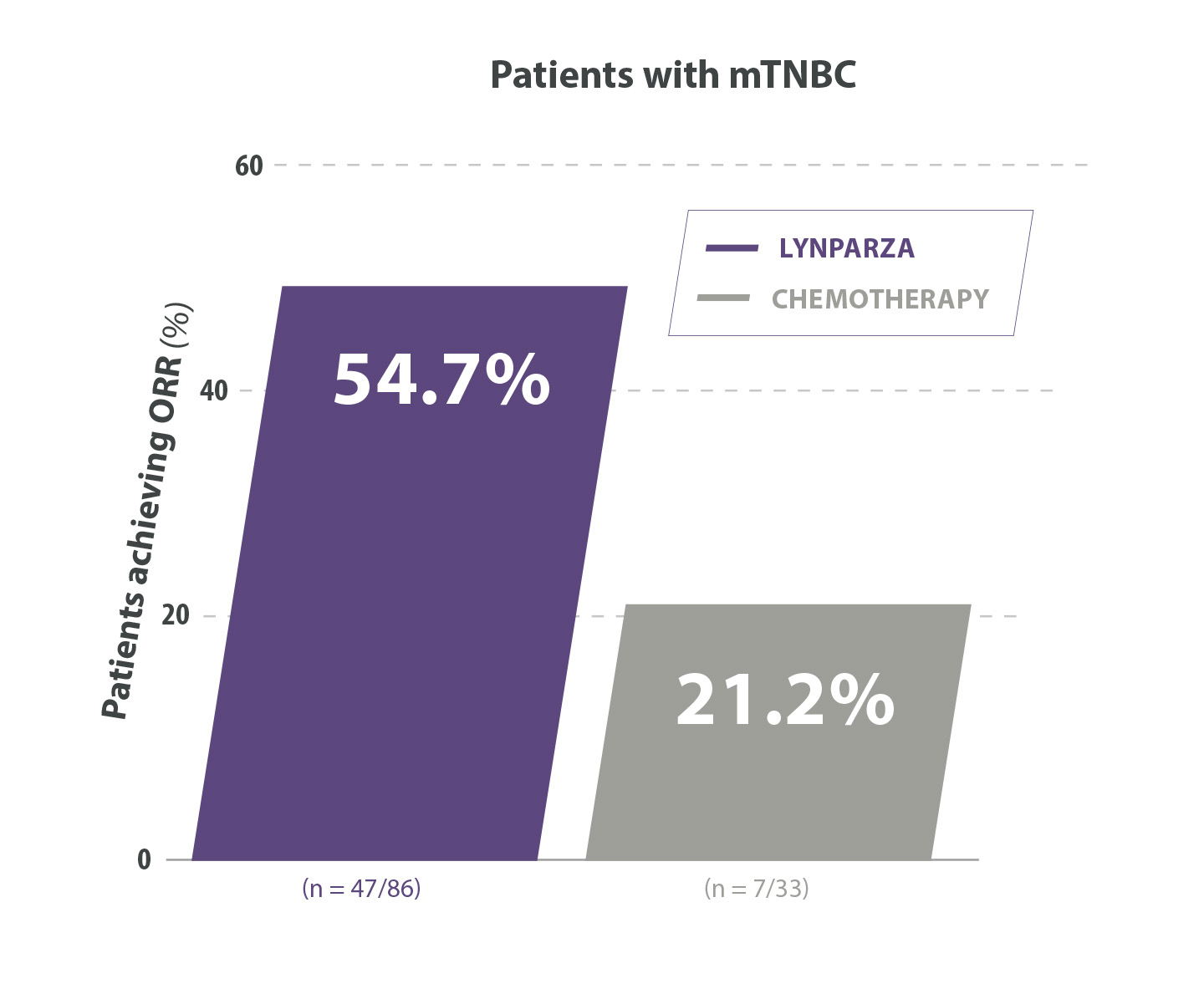
Lynparza®verdubbelde de ORR vs placebo in de TNBC-subgroep11
Adapted from Robson M et al. 2017. Supplementary appendix.
ORR=objective response rate ; TN=triple negative
Veiligheidsprofiel
Vroege borstkanker (OlympiA studie)
Bijwerkingen gerapporteerd bij ≥10% van de patiënten in de OlympiA studie*6
Adapted from Tutt ANJ et al. 2021.
*All listed are Grade 3 except for 10 Grade 4 events in the LYNPARZA group: Five events involving decreased neutrophil count, four involving anaemia, and one involving fatigue. AE=adverse event; AML=acute myeloid leukaemia; MDS=myelodysplastic syndrome
Ongeveer 70 % van de bijwerkingen met Lynparza® was graad 1 en 2.12
Er was geen significant verschil in de frequentie van myelo-dysplastisch syndroom (MDS) of acute myeloide leukemie (AML) met Lynparza® in vergelijking met placebo (0.2% met Lynparza® vs. 0.3% met placebo).6
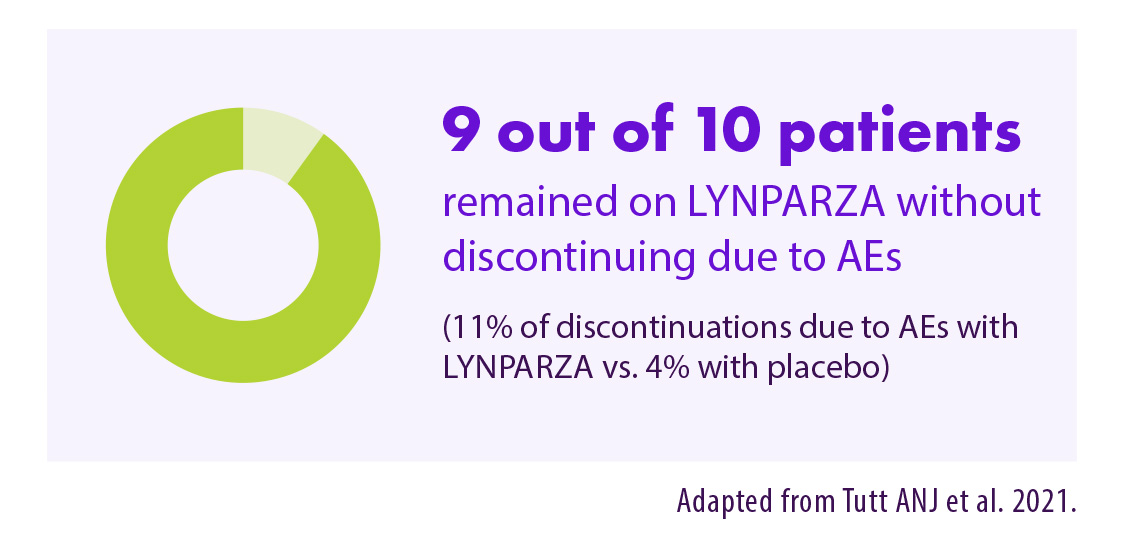
90% van de patiënten bleef Lynparza® gebruiken zonder onderbreking wegens bijwerkingen12
Adapted from Tutt ANJ et al. 2021. Supplementary appendix
Lokaal gevorderde of gemetastaseerde borstkanker (OlympiAD studie)
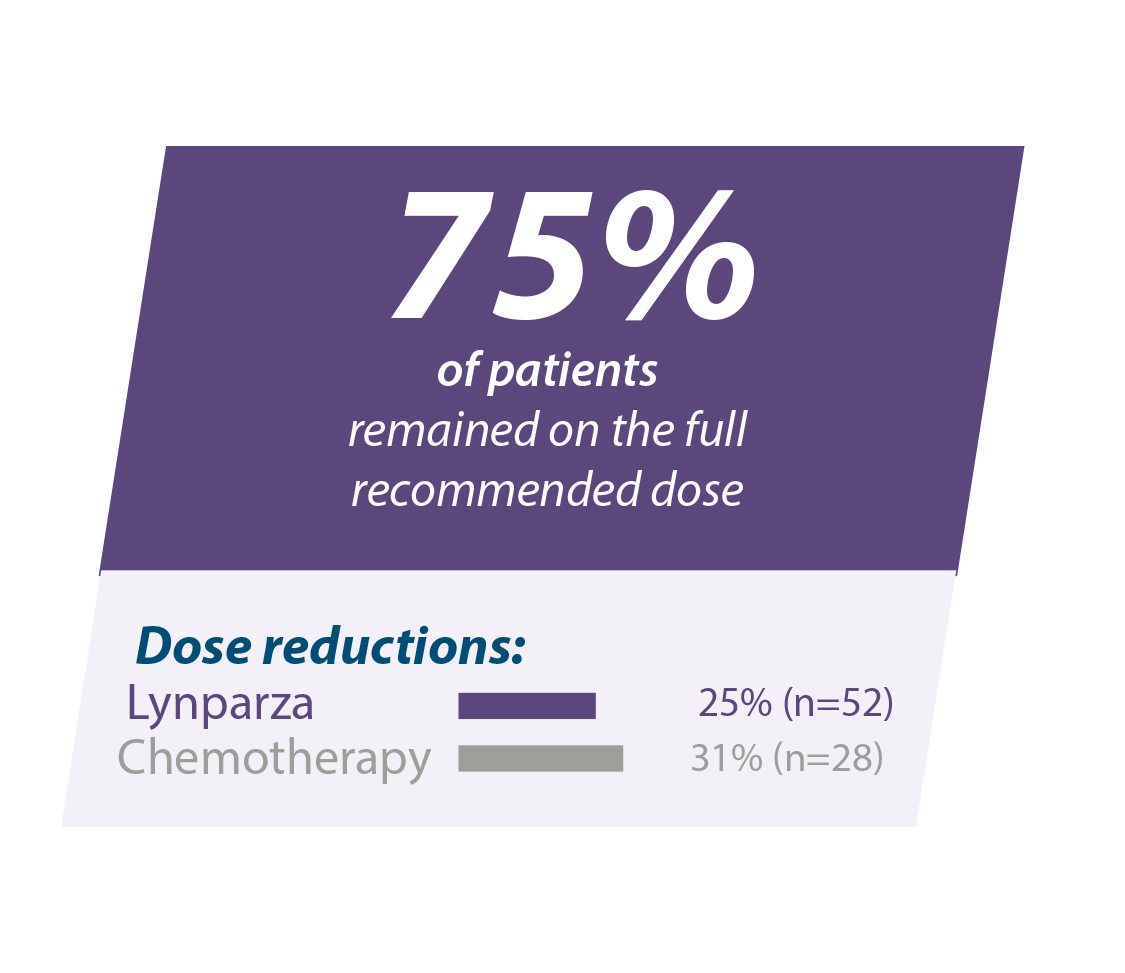
De bijwerkingen in OlympiAD waren meestal mild tot matig van ernst en beheersbaar door onderbreking of vermindering van de dosis.10
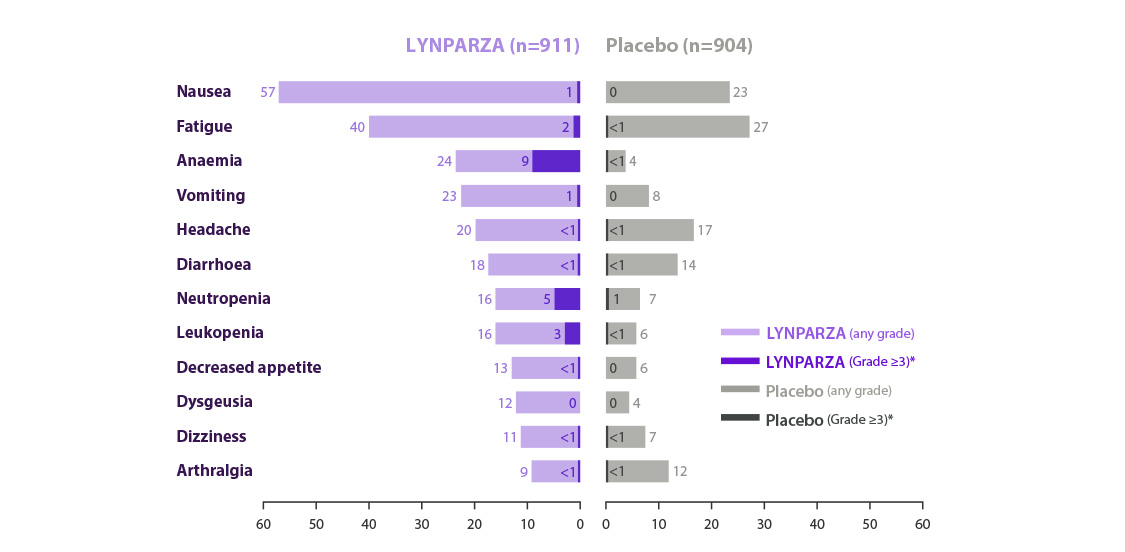
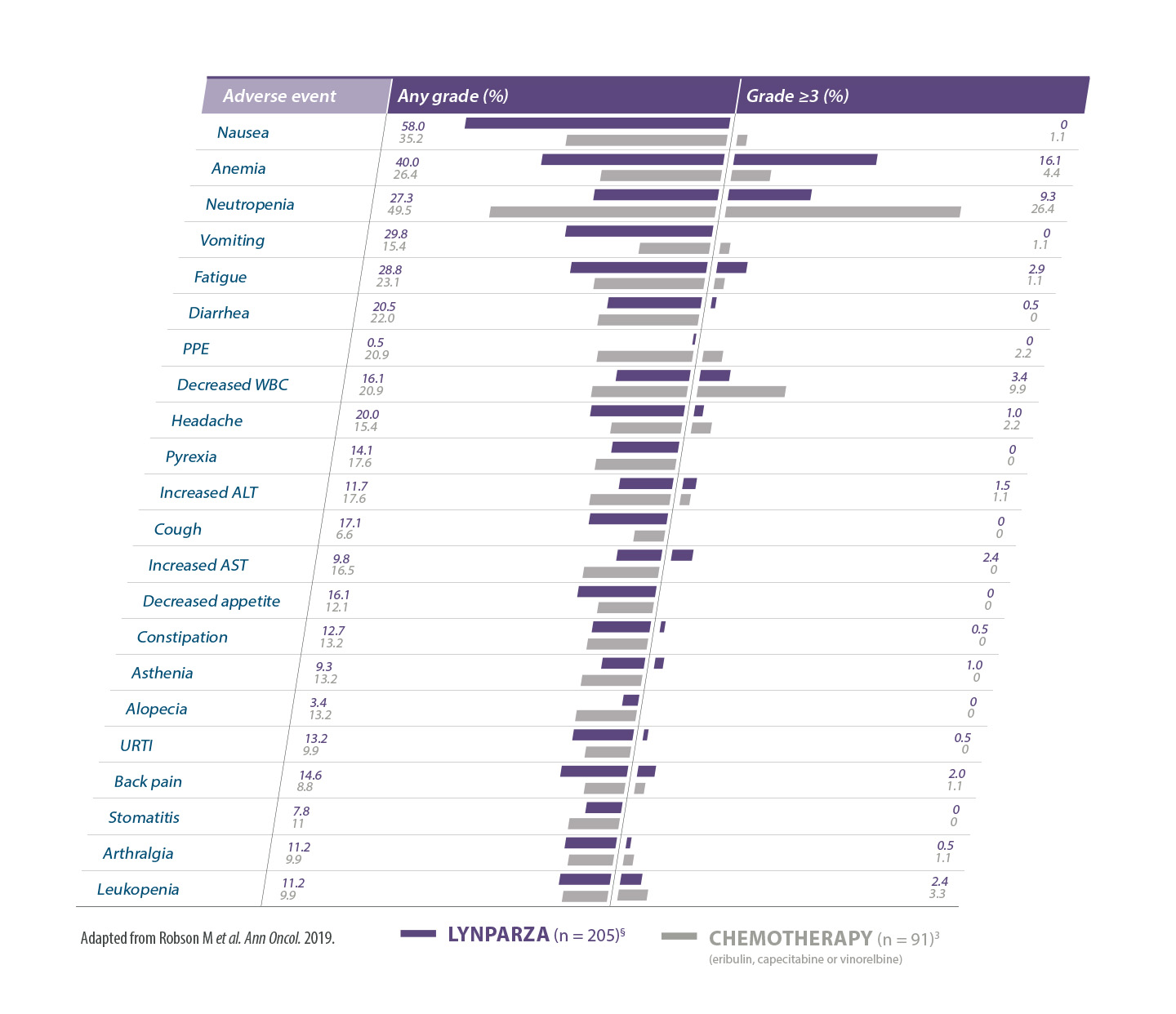
Adverse events reported in ≥15% of patients in OlympiAD*10
AEs of any cause; MedDRA-preferred terms are grouped for anemia (anemia, decreased Hb level, decreased hematocrit, decreased red blood cell count, and erythropenia) and neutropenia (febrile neutropenia, granulocytopenia, decreased granulocyte count, neutropenia, neutropenic sepsis, decreased neutrophil count, and neutropenic infection). ALT= alanine aminotransferase; AST= aspartate aminotransferase; PPE=palmar plantar erythrodysesthesia;.URTI=upper respiratory tract infection; WBC=white blood cells.
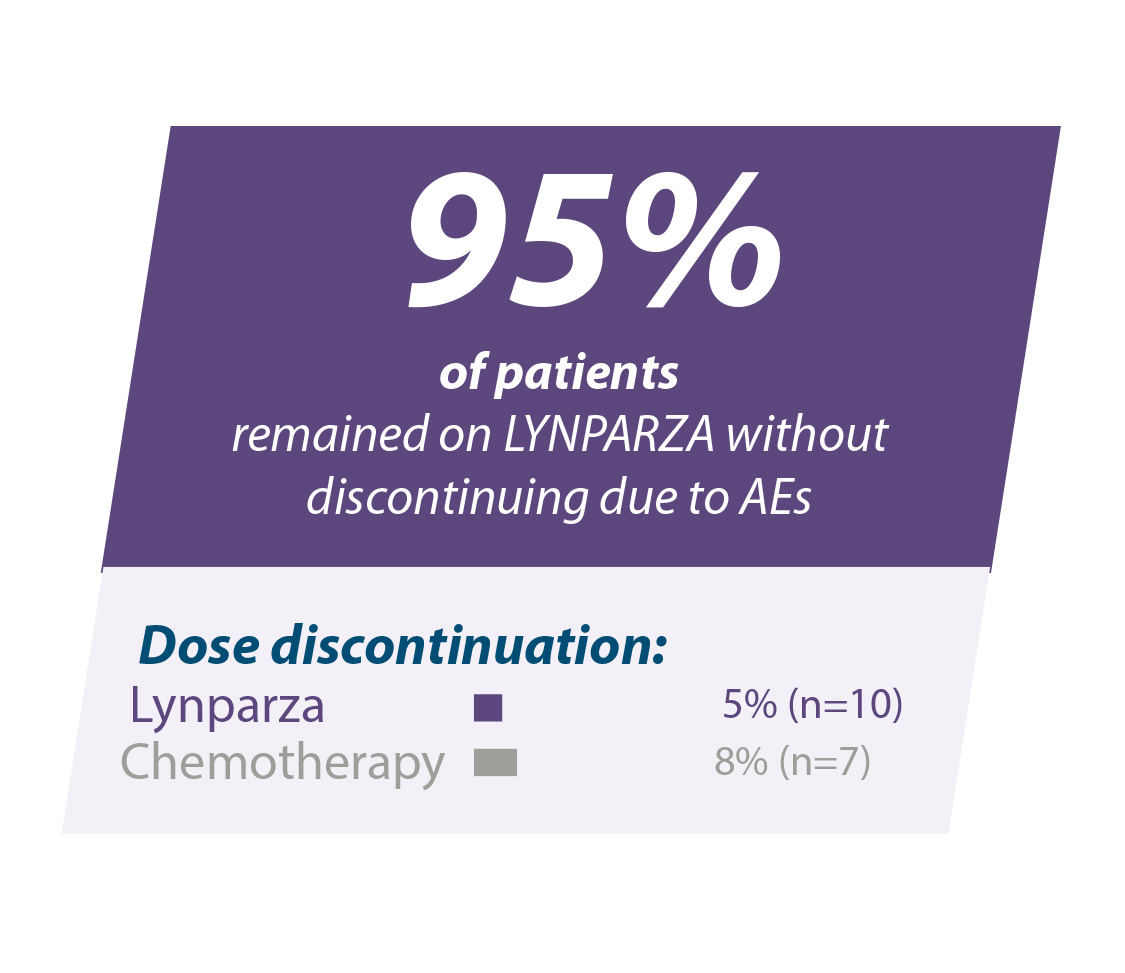
95 % van de patiënten bleef Lynparza® gebruiken zonder onderbreking wegens bijwerkingen10
Onderzoek naar genetische mutaties
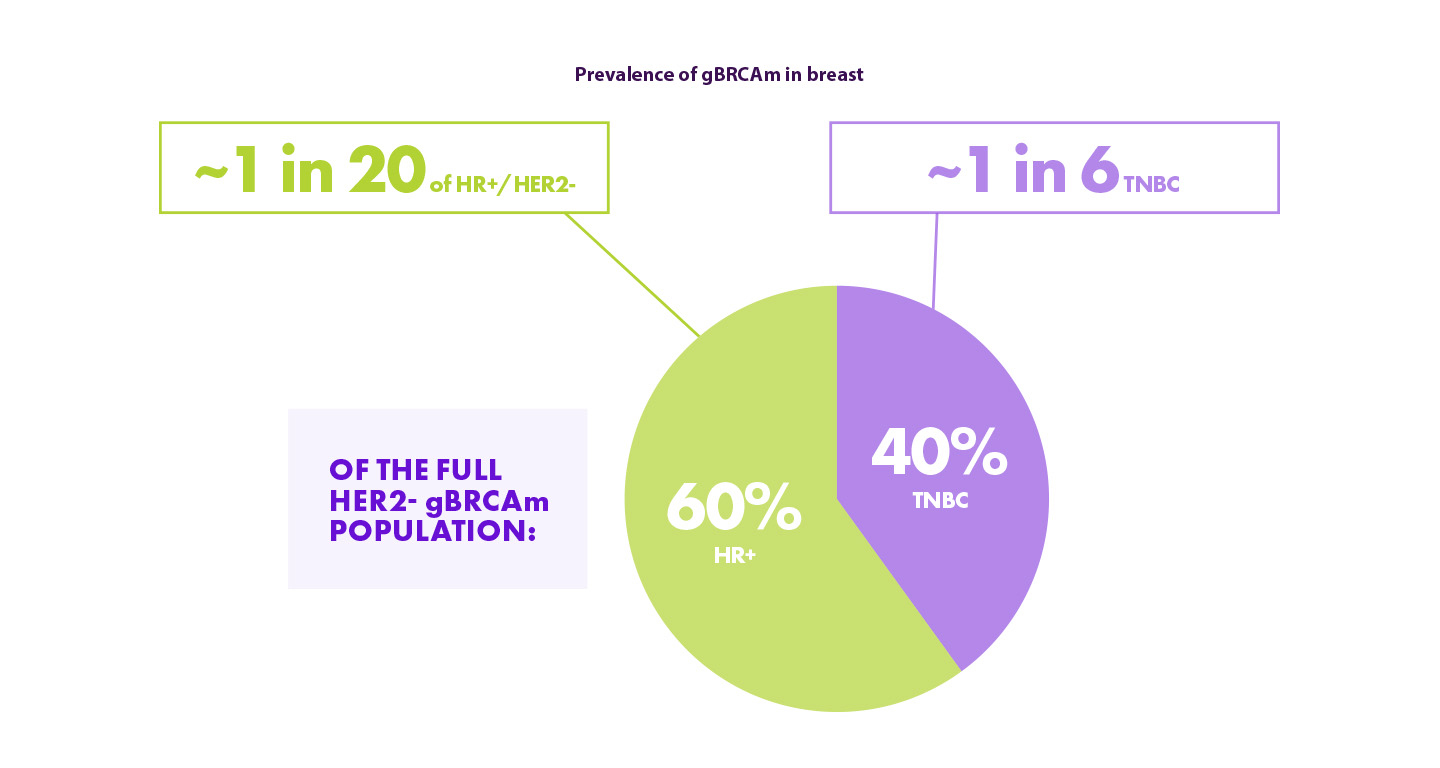
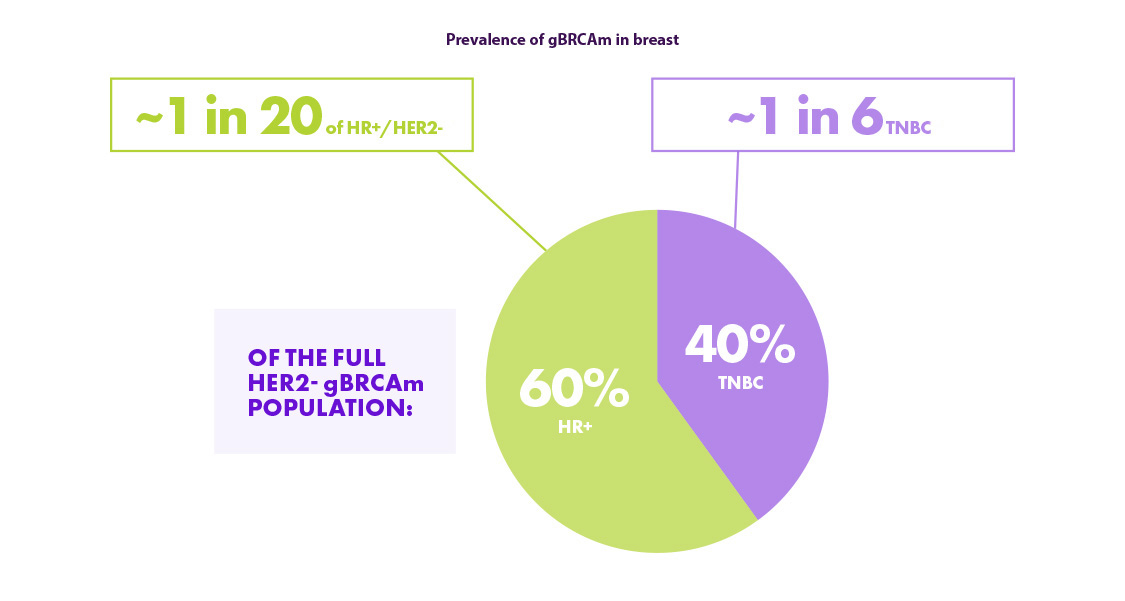
Hoewel de prevalentie van gBRCA in TNBC hoger is dan in HR+/HER2-, is de meerderheid van de HER2- gBRCAm-patiënten HR+ omdat de HR+/HER2- subgroup groter is. 13,14
Hierdoor is het belangrijk om HR+ patiënten niet over het hoofd te zien wat betreft de screening voor gBRCA mutaties.
gBRCAm = germline BRCA-mutated; HER2 = human epidermal growth factor receptor-2; HR+ = hormone receptor-positive; TNBC = triple-negative breast cancer.
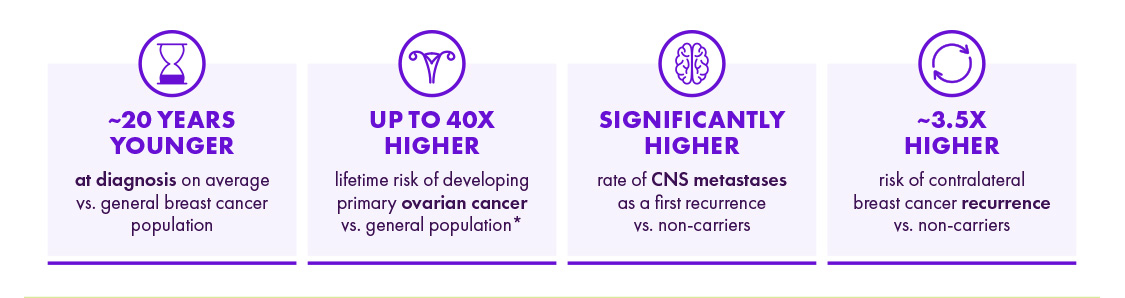
gBRCA is een biomarker van een agressievere ziekte met een slechtere prognose15-20
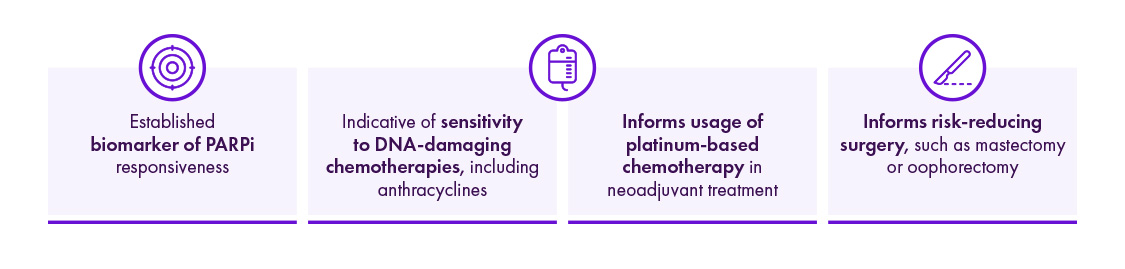
BRCA-tests bij diagnose zijn essentieel om zowel de behandeling als de chirurgische aanpak te bepalen17,21-26
CNS = central nervous system; gBRCA = germline BRCA; DNA = deoxyribonucleic acid; PARPi = polymerase inhibitor.