Prostaatkanker
Over Lynparza
Werkingsmechanisme
Olaparib is een sterke remmer van menselijke poly (ADP-ribose) polymerase-enzymen (PARP-1, PARP-2 en PARP-3) en het is aangetoond dat dit middel de groei van bepaalde tumorcellijnen in vitro en de groei van tumoren in vivo remt, ofwel als opzichzelfstaande behandeling of in combinatie met gevestigde chemotherapieën.1
Samenvattend: BRCAm tumoren hebben een unieke moleculaire biologie die hen gevoelig maakt voor PARP-remming.3,4
Lynparza remt PARP op twee manieren:†3-4
- Door de enzymatische activiteit te remmen3-4
- Door de vorming van gevangen PARP-DNA complexen te verhogen3-4
Samenvatting van de resultaten
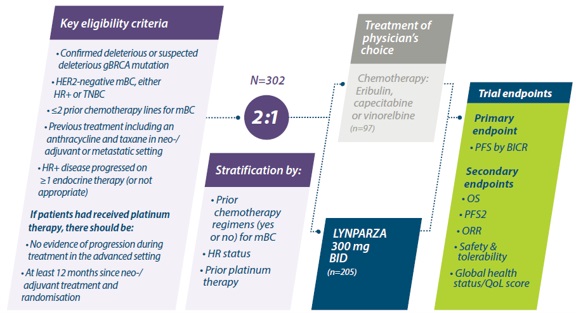
PROfound: De eerste gerandomiseerde, gecontrolleerde, fase III studie van een PARPi in prostaatkanker5,6
*Determined by prospective tumour tissue testing – 15-gene panel.5
†Three patients in the LYNPARZA® arm in Cohort A had co-occurring mutations in both BRCA and ATM.7
‡Patients retreated with a different NHA; physician’s choice of either enzalutamide (160 mg QD) or abiraterone (1000 mg QD) + prednisone (5 mg BID).5
¶rPFS according to RECIST 1.1 & PCWG3 by BICR. ORR in evaluable patients with measurable disease at baseline, according to RECIST 1.1 by BICR5
§Key secondary endpoints were included in hierarchical testing for statistical significance.6,11
ATM = ataxia-telangiectasia mutated; BICR=blinded independent central review; BID=twice daily; BRCA = Breast cancer gene; HRR=homologous recombination repair; mCRPC=metastatic castration-resistant prostate cancer ; NHA=new hormonal agent; ORR=objective response rate; OS=overall survival; PARPi=poly (ADP-ribose) polymerase inhibitor; PCWG3=Prostate cancer Working Group 3; QD=once daily; RECIST=Response Evaluation Criteria in Solid Tumors; rPFS=radiographic progression-free survival.
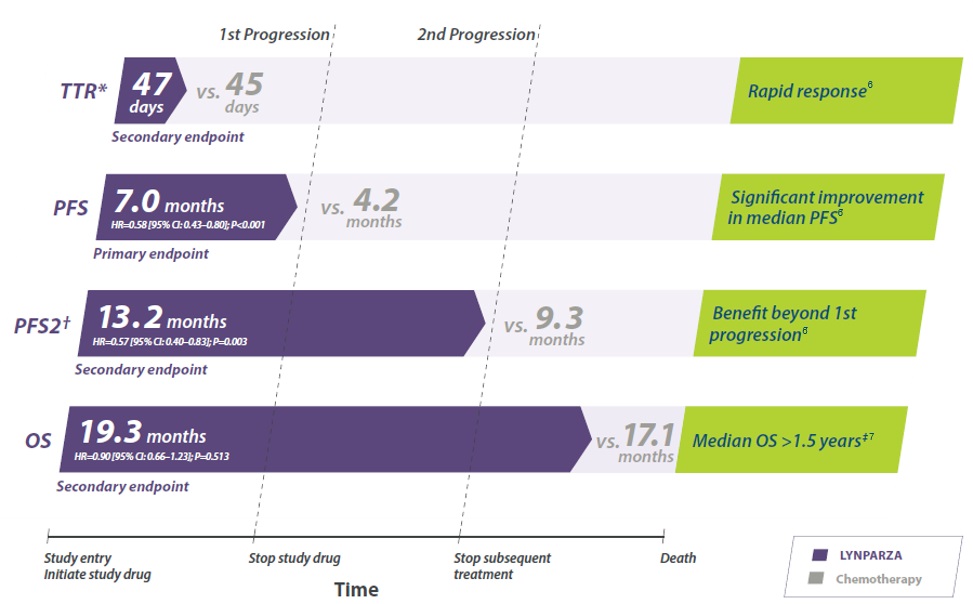
Overall survival in BRCA gemuteerde patienten1,5
Data presented for 160 BRCA-mutated patients. 141 had single mutations in BRCA1 or BRCA2; 19 had BRCA1/2 and co-occurring mutations in other HRR genes.9
*The HR and CI were calculated using a Cox proportional hazards model that contains terms for treatment, factor and treatment by factor interaction. rPFS 71% maturity at data cut off.1
BRCA=breast cancer gene; BRCA1=breast cancer gene 1; BRCA2=breast cancer gene 2; CI=confidence interval; HR=hazard ratio; NHA=new hormonal agent; ORR=objective response rate; rPFS=radiographic progression-free survival.
Data presented for 160 BRCA-mutated patients. 141 had single mutations in BRCA1 or BRCA2; 19 had BRCA1/2 and co-occurring mutations in other HRR genes.9
*The HR and CI were calculated using a Cox proportional hazards model that contains terms for treatment, factor and treatment by factor interaction. rPFS 71% maturity at data cut off.1
BRCA=breast cancer gene; BRCA1=breast cancer gene 1; BRCA2=breast cancer gene 2; CI=confidence interval; HR=hazard ratio; NHA=new hormonal agent; ORR=objective response rate; rPFS=radiographic progression-free survival.
Radiografische progressie vrije overleving in BRCA gemuteerde patienten1,5
Data presented for 160 BRCA-mutated patients. 141 had single mutations in BRCA1 or BRCA2; 19 had BRCA1/2 and co-occurring mutations in other HRR genes.2
*The HR and CI were calculated using a Cox proportional hazards model that contains terms for treatment, factor and treatment by factor interaction. rPFS 71% maturity at data cut off.1
BRCA=breast cancer gene; BRCA1=breast cancer gene 1; BRCA2=breast cancer gene 2; CI=confidence interval; HR=hazard ratio; NHA=new hormonal agent; ORR=objective response rate; rPFS=radiographic progression-free survival.
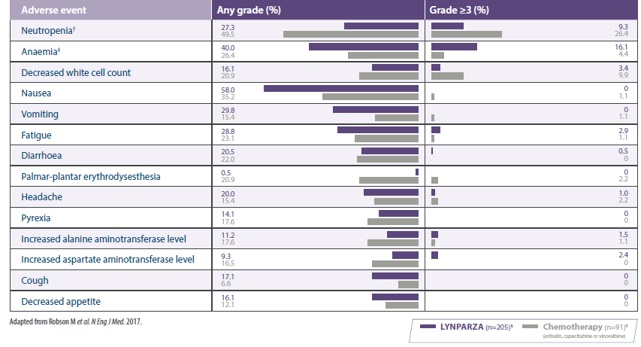
Veiligheidsprofiel
Het veiligheidsprofiel dat werd waargenomen in PROfound5,10 was in overeenstemming met het algemene veiligheidsprofiel van LYNPARZA.®1
Nevenwerkingen gerapporteerd in ≥10% van patiënten in PROfound1,5,10
Pulmonary embolism occured in 5% of patient with Lynparza® vs 1% with NHA retreatment, none were fatal10
Safety data from PROfound is presented for the overall population, including BRCA1/2, ATM and 12 other HRR mutations. LYNPARZA® should only be prescribed in the appropriate indicated population.1,10
*Includes anaemia, decreased haemoglobin level, decreased red cell count, decreased haematocrit level, erythropaenia, macrocytic anaemia, normochromic anaemia, normochromic normocytic anaemia, and normocytic anaemia. Anaemia was reported in 49% of the patients, and a decreased haemoglobin level was reported in less than 1%.10
†One patient in the control group did not receive treatment.10
AE=adverse event; ATM=ataxia-telangiectasia mutated; BRCA1=breast cancer gene 1; BRCA2=breast cancer gene 2; HRR=homologous recombination repair; NHA=new hormonal agent.
Genetische testing
Klik hier voor een gedetailleerde infographic omtrent genetische testing.
Tumor testing* is reimbursed for mCRPC patients
- Germline testing is recommended in patients with a family history of cancer and should be considered in all patients with metastatic prostate cancer
- *If BRCA mutations in tumour are found, refer to genetic counselling for confirmatory germline testing.11,12
BRCA=breast cancer gene; BRCA1=BReast CAncer gene 1; BRCA2=breast cancer gene 2; mCRPC=metastatic castration-resistant prostate cancer; NHA=new hormonal Agent
.jpg)